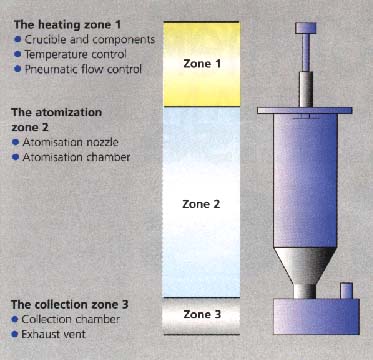
| ethylenes studied thus
far, it has been found that the formation of whiskers and elongated spheres
can be avoided by using high gas atomisation pressures (approximately 7.6MNm-2),
fig 3. It appears that a mixture of whiskers and spheres would be ideal
for making self-reinforced polymer powders that can be used for applications
requiring improved mechanical properties. Investigations on expanding the
employment of GAP to other polymers with vastly different thermal and rheological
properties are continuing. More recently, 50/50 blends of PE130 and ultra-low
melting polyphosphate glass composition have been successfully atomised
under conditions that were used to atomise the pure PE130 polymer. This
result confirms the expectation of the broad application of GAP to many
fields, such as in producing polymer alloys, glass-polymer alloys, in situ
composites, and related products with tailored properties for beneficial
uses in many areas such as decorative or protective coatings, polymer-supported
heterogeneous catalysts, and in producing lightweight structural composites.
The structural composites can be easily fabricated by applying established
solidstate powder compaction methods to the gastomised composite powders
to form compacts with varying shapes and sizes. The research conducted thus
far has provided valuable insight into GAP diagnostic control systems, dynamics
and mechanisms of powder formation. This knowledge can be used to expand
the application of the method to include the production of other kinds of
materials with desirable properties for beneficial uses. The desirable properties
of the powders include the following: purity; particle shape; particle size;
and size distribution. The many teething problems of GAP when it is applied
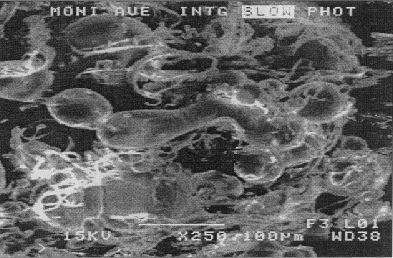
to |
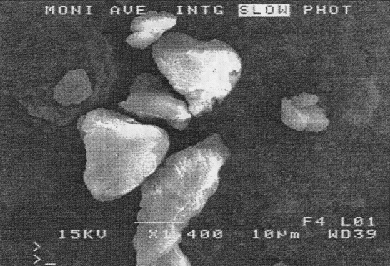 |
| polymers and composites
are now understood and can be controlled and managed in order to produce
powders with tailored characteristics. The technology of GAP has now advanced
to a stage of finding more applications in the areas of polymer engineering,
composite engineering, and of scaling-up to mass production of the fine
polymer powders |
Figure
3 SEM of commercially ground PE based wax (top), PE520 atomised at pressures
of 7.6MNm-2 (above) and 2MNm-2 (below). (the PE520 was atomised at a temperature
of 190 C |
|
Acknowledgements
The authors thank Iver Anderson, Ames Laboratory, Hoechst-Celanese and
Allied Signal Inc without whose support and encouragement this work would
not have been possible. The computer simulation contribution of Don Noid,
RE Tuzun, and Bobby Sumpter (Oak Ridge National Laboratory) to this work
is thankfully acknowledged. Financial support of this work by the Center
for Advanced Technology Development and Iowa State University is grateful
acknowledged.
|
 |
|
Further reading
JIM McAvoy, JU Otaigbe, Gas Atomisation of polymers, SPE ANTEC' Tech
Papers, 1997, 43 2071. RE Tuzun, DW Noid, BG Sumpter, JU Otaigbe Proc
ACS: Div Polym Mat Sci, 1997, 76, 585. JU Otaigbe, JM McAvoy, IE Anderson,
US patent application 09-0530, filed 17 July, 1996. JP Jog, Adv Polyrn
Tech, 1993 , 12,281-293.
|